Stem Cells: Promises and Deceptions
Stem cells are essential for the success of the in vitro tissue engineering endeavor. Whether in building in vitro tissue models for drug screening or in creating tissue grafts for applications in regenerative medicine, the use of stem cells opens up possibilities which are impractical or even impossible with other cell types. Unfortunately, stem cells have also become part of the sales pitch of numerous companies and clinics offering treatments which may not be evidence-based.
Why are stem cells so important? One of the reasons lies in their high in vitro proliferation potency which is in contrast to the poor proliferative ability outside the human body of terminally differentiated cells. This fact adds to the well known weak ability for regeneration of some of the organs and tissues, such as the brain, the cartilage, the heart muscle, and others. The cells’ ability to renew and multiply is important for in vitro tissue and organ engineering because the number of cells in a typical bioprinted “tissue” is up to about a million while there are billions of cells within the heart, for example. However, even with the help of highly proliferative cells, generating this number of new cells is still a major, unresolved challenge facing the entire 3D-bioprinting and tissue engineering fields. The challenge is related to the difficulty to reach high cell density while ensuring that all cells in an in vitro tissue have access to sufficient amount of nutrients and effectively exchange gas and other byproducts of their normal functioning, required for their survival and the maintenance of their identity. As a result, the research into recreating the vascular architecture of a native tissue or simply creating an effective alternative is an active and vital area of investigation which we will review in a future blog post.
What is a stem cell? The most distinguishing characteristic of a stem cell is its ability to develop into many different cell types. Into how many? The answer to that question divides the stem cells into totipotent (all), pluripotent (many), multipotent (several), oligopotent (few), and unipotent (one). The focus of this article will be on a class of multipotent cells but we will briefly discuss the various types of stem cells.
The only totipotent stem cells are the blastomeres, cells produced by cleavage of a fertilized egg. They are also called embryonic stem cells (ESC). The use of ESCs for research has led to ethical and political controversies which is why many research groups focus on the other types of stem cells. An important thing to note here is that the source of the ESCs are eggs fertilized at in vitro fertilization clinics but never implanted into a woman’s uterus.
A notable category of the pluripotent cells are the induced pluripotent stem cells or iPSCs. In 2012, Shinya Yamanaka was awarded the Nobel Prize for Physiology or Medicine for his discovery that mature skin cells can be converted into pluripotent stem cells, called iPSCs. The use of iPSCs in tissue engineering is steadily increasing and has already resulted in reports of vascularized heart tissue as well as skeletal muscles and muscular dystrophies models.
The naturally occurring adult stem cells are generally restricted to limited sets of cell lineages, i.e. they are uni-, oligo- or multipotent, which constrains their therapeutic potential. Nevertheless, there is still considerable interest in understanding the processes by which adult stem cells orchestrate tissue growth and development. The unipotent stem cells are distinct from differentiated cells in that they can divide repeatedly which is the mechanism by which the adult tissues can maintain certain capacity to regenerate. On the other hand, typical examples of oligopotent stem cells are the myeloid and lymphoid stem cells which give rise to all the blood cells.
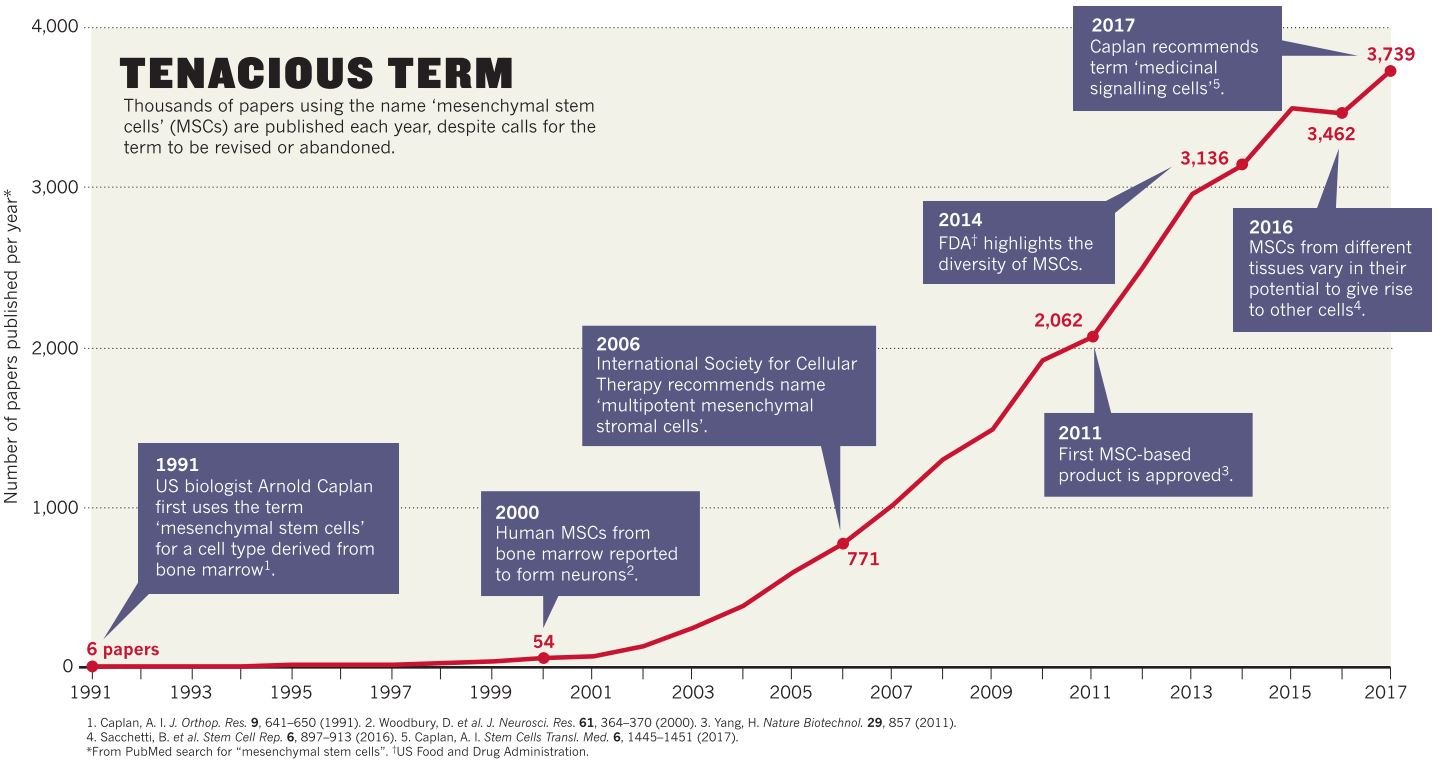
Credit: Sipp et al. (2018)
In contrast, a class of multipotent cells, the MSCs, has become one of the most contentious topics in science. It has led to commentaries in the journals Science and Nature with expressive titles like “Confronting stem cell hype” and “Clear up this stem-cell mess”. The best illustration of the issue is probably the fact that their original and well-established name Mesenchymal Stem Cells is recommended to be avoided by both The International Society for Cellular Therapy (ISCT), today The International Society for Cellular and Gene Therapy, and the father of MSCs, Arnold Caplan. As an alternative, they have suggested the names Multipotent Mesenchymal Stromal Cells and Medicinal Signaling Cells, respectively. ISCT’s recommendation was followed by strict criteria which need to be met before specific cells can be classified as MSCs. However, it seems that even if majority experts agree with these criteria, there is not really a consensus. Importantly, it has also been established that often MSC differentiation is not the mechanism by which they facilitate tissue regeneration. Instead, MSCs might induce the differentiated cells and stimulate their capacity to proliferate but the MSCs might ultimately disappear without differentiating into the target cells. Furthermore, the evolving insight of the MSC’s nature has established that most of them, if not all, are derived from pericytes, vascular cells embedded in the basement membrane of blood microvessels, which has challenged the “stromal” origin of the MSCs. Nevertheless, these cells remain of clinical interest for their clear ability to modulate the immune system as well as their potential to regenerate tissues.
Unfortunately, there are businesses with dubious ethical standards which take advantage of this landscape of incomplete scientific knowledge. For instance, a report documenting 351 US companies selling stem-cell treatments direct to consumers of which almost half refer to MSCs in their advertising materials raises concerns regarding the scientific evidence on which these claims are based. In this context, it is often crucial that knowledgeable parties organize and maintain informational campaigns tailored to protect the public from advertising of unproven treatments.
Fortunately, experts like Timothy Caulfield, Canada research chair in health law and policy at the University of Alberta, have made it their mission to organize physicians, advertising regulators and governmental agencies to protect the public from the advertising of unproven stem cell therapies and the so called stem cell tourism.
Ultimately, stem cells are and will remain instrumental to the progress and the success of the field of tissue engineering. Their proliferative capacity as well as their ability to stimulate already differentiated cells to proliferate are essential in bridging the gap between the number of cells used in a typical in vitro experiment and the number required to model a real life tissue or organ. In the meantime, we need to be skeptical of and demand proper evidence for the typically quite expensive “stem cell treatments” we are increasingly likely to be offered.